Genethon’s DNA and Cell bank aims to boost progress in genetic research by providing the scientific community with the high-quality services of a cell and human products bank. Europe’s leading bank for genetic diseases, it operates as a service to the entire medical and scientific community. It is certified according to the AFNOR NF S96-900 standard.
Genethon’s DNA and Cell bank performs the following annually:
- 2,000 lymphoblastoid cell lines
- 3,000 DNA extractions
- Primary cultures of myoblasts and fibroblasts of around 100 biopsies
Total storage capacity: 436,000 samples
Activities of the DNA and Cell bank

The bank’s mission is exclusively research-based and it has no diagnostic role. It is open to all researchers in France or overseas wishing to store samples, or utilize services (such as extraction, establishment of lines, etc.).
Each sample received at Genethon is coded to guarantee anonymity of the donor, in accordance with the rules set out by the French data protection authority (CNIL).
List of services offered by the DNA and Cell Bank
- Collect blood or DNA of patients suffering from genetic diseases and their families with the minimum identification required to check sample follow-up.
- Process samples to make them available to the scientific community, and ensure durable DNA preservation (isolation of the serum, DNA extraction), isolate lymphocytes and establish lymphoblastoid B cell lines, and primary cultures (mainly myoblasts and fibroblasts).
- Store samples for future research and to preserve genetic heritage, ensuring long-term physical integrity of the samples stored.
- Distribute the samples needed for current research in accordance with the principles and laws of bioethics, using the best technologies at the lowest cost.
All these activities are carried out according to Standard Operating Procedures (SOP) validated by Genethon’s Quality Assurance department.
Collaborate with the DNA and Cell bank: how it works
All collaborations with the DNA and Cell Bank require a written request addressed to Dr. Safaa Saker-Delye.
Sending biological resources
All requests to send, process and store samples are covered by a collaboration agreement specifying the rights and obligations of the parties.
Services are provided in accordance with the pricing scale.
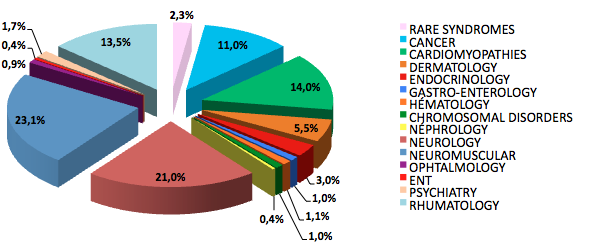
| Group of pathologies | Number of individuals | Number of families |
| Cardiomyopathy | 8,933 | 4,172 |
| Dermatology | 6,599 | 1,146 |
| Endocrinology | 1,438 | 722 |
| Gastroenterology | 214 | 153 |
| Hematology | 663 | 174 |
| Chromosomic diseases | 434 | 286 |
| Nephrology | 309 | 71 |
| Neurology | 17,277 | 6,290 |
| Neuromuscular diseases | 17,921 | 8,731 |
| Ophtalmology | 487 | 130 |
| Otorhinolaryngology | 327 | 62 |
| Psychiatry | 1,223 | 265 |
| Pneumology | 4,885 | 4,860 |
| Rhumatology and bone diseases | 5,521 | 1,560 |
| Rare syndromes [in French] | 2,157 | 726 |
| Total | 68,388 | 29,348 |
June 2019
Catalog of myoblasts [in French]
DNA and Cell bank: management and traceability
Management and traceability of samples is achieved via a computer database developed at Genethon. This base has been declared and authorized by the CNIL. Genethon’s DNA and Cell bank is certified according to the CRB AFNOR NF S 96-900 standard.
Charter and instructions for sampling and delivery of biological resources
A charter governs relations between the DNA and Cell Bank and the individuals using its services, in accordance with the ethical principles governing the collection of human products, the legislative and regulatory provisions governing the sampling, processing and storage of said products and the related information.
The DNA and Cell Bank follows the Instructions for sampling and transport of biological resources specified.
Partners
Genethon’s DNA and Cell bank is an IBiSA platform. As such, it collaborates with a number of partners.
- Institut national de la santé et de la recherche médicale (Inserm)
- Centre national de la recherche scientifique (CNRS)
- Patient associations (RETT syndrome, Anti-autism foundation, etc.)
- Neuromuscular disease research networks supported by AFM-Telethon.
- Cancer research centers (Institut Curie, Gustave-Roussy)
- Partnerships with scientists and institutions in countries in the Mediterranean and the Middle East on rare diseases (Algeria, Tunisia, Morocco, Turkey, Lebanon, Syria, Iran and Saudi Arabia)
- Partnerships within European projects: European consortium for research on rheumatoid arthritis (ECRAF), European research project on ankylosing spondylarthritis (EuroAs), EuroBioBank (European network of banks for research on rare diseases), European Research Infrastructure Bio-Banking and Biomolecular Resources (BBMRI) and Treat-NMD.

